Chemical Processes
Powder detergents are manufactured using various processes, such as spray drying, agglomeration, dry mixing or a combination of these. A brief description of these different processes is given below -
Spray Drying Process
The different stages / operations performed in a spray drying process, are -
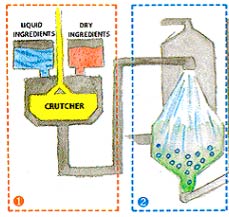
- Dry and liquid ingredients are first combined into a slurry, or thick
suspension, in a tank known as crutcher.
- The slurry is heated and then pumped to the top of a tower where it
is sprayed through nozzles (under high pressure) to create small
forming hollow granules as they dry.
droplets. The droplets fall through a current of hot air, thereby
- Collected from the bottom of the spray tower, the dried granules are
screened to obtain a relatively standard size.
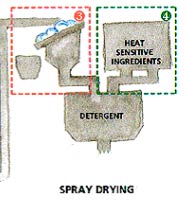
- After the granules have been cooled, heat sensitive ingredients, which are not compatible with the spray drying temperatures (like bleach, enzymes and fragrance) are added.
 Traditional
spray drying process produces relatively low-density detergent powders.
Advancements in technology have enabled the soap and detergent manufacturers
to reduce the air inside the granules during spray drying to obtain higher
densities. The high-density detergent powders can be packed in much smaller
packages than those needed previously.
Traditional
spray drying process produces relatively low-density detergent powders.
Advancements in technology have enabled the soap and detergent manufacturers
to reduce the air inside the granules during spray drying to obtain higher
densities. The high-density detergent powders can be packed in much smaller
packages than those needed previously. Agglomeration
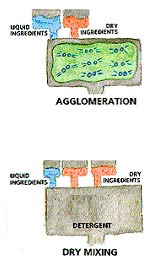
Agglomeration is detergent powder manufacturing chemical technique that results in high-density powders. The process involves blending of dry raw materials with liquid ingredients. The technique involves machines, such as a liquid binder, rolling or shear mixing that causes the ingredients to collide and adhere to each other, producing larger particles.
Dry Mixing
Dry mixing is a detergent powder manufacturing technique, which is used to blend dry raw materials. The technique may also involve the addition of small quantities of a liquid.
Ingredients
Mentioned in the table below are the ingredients of a detergent base powder -
| Solids | |
| Ingredient | Function |
| Sodium tripolyphsophate(STP) | Water softener, pH buffers (to reduce alkalinity). |
| Sodium sulphate | Bulking and free-flowing agent. |
| Soap noodles | Causes rapid foam collapse during rinsing. |
| Zeolite | Water softener (absorbs Ca 2+ and Mg 2+) in countries where STP is not used; granulating agent for concentrated detergents. |
| Sodium carboxymethyl cellulose | Increases the negative charge on cellulosic fibers, like cotton and rayon, causing them to repel dirt particles (which are positively charged). |
| Liquids | |
| Ingredient | Function |
| Linear alkylbenzene sulphonic acid (LAS) | Surfactant - the main active ingredient |
| Caustic soda solution | Neutralizes the LAS. |
| Coconut diethanolamide or a fatty alcohol ethoxylate | Nonionic detergent and foam former. |
| Fluorescer | Absorbs UV light and emits blue light, causing aging cotton to appear white rather than yellow. |
| Water | Dissolves the various ingredients, causing them to mix better. |
Environmental Implications
Detergent powder manufacturing has some specific environmental issues that are not associated with any other industry. These issues include dust control and volatile organic emissions. Dust present during the process of delivery and transfer of bulk powdered detergent is a potential problem. In order to filter out most of the dust, dry and wet cyclones are used, and all the emissions are monitored.
If the dust level in these does exceeds the acceptable limits, appropriate remedial action must be taken. The permissible dust levels in emissions are under below 50-mg m -3. The spray-drying tower also releases volatile organics, which can be minimized by having tight specifications specifying what can be added as a primary detergent active material. Spot checks must be made on the total hydrocarbon content of the exhaust gases using a flame ionization detector.

